OEM manufacturer Disposable For Biopsy Forceps - Disposable cytology brush – Chenmao
OEM manufacturer Disposable For Biopsy Forceps - Disposable cytology brush – Chenmao Detail:
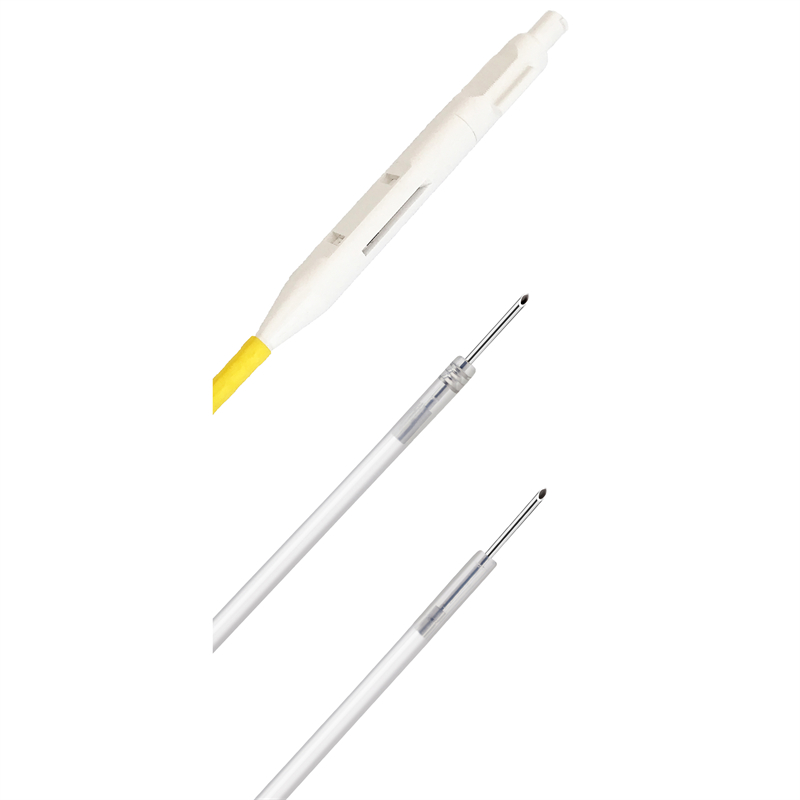
Usage
Injection needle is intended for submucosal injection in digestive tract combined with endoscope.
Advantage
Concealed needle design ,effectively avoiding damages to the working channel.
Transparent needle tube,excellent control of drugs and fluids.
Excellent lubrication performance of outer tube ,smoothly passing through the channel .
Classic handle design,the injection points are clear .
Discount price for sample order

Specifications
|
Model |
O.D. (mm) |
Working Length (mm) |
Needle model |
Needle Length (mm) |
Endoscopic Channel (mm) |
Characteristic |
| GM-IN-A-23-1600-23-4 |
2.3 |
1600 |
23G |
4 |
≥2.8 |
Without metal cap |
| GM-IN-A-23-2000-23-5 |
2.3 |
2000 |
23G |
5 |
≥2.8 |
|
| GM-IN-A-23-2300-23-5 |
2.3 |
2300 |
23G |
5 |
≥2.8 |
|
| GM-IN-A-23-2300-23-6 |
2.3 |
2300 |
23G |
6 |
≥2.8 |
|
| GM-IN-B-23-1600-23-4 |
2.3 |
1600 |
23G |
4 |
≥2.8 |
With metal cap |
| GM-IN-B-23-2000-23-6 |
2.3 |
2000 |
23G |
6 |
≥2.8 |
|
| GM-IN-B-23-2300-23-5 |
2.3 |
2300 |
23G |
5 |
≥2.8 |
|
| GM-IN-B-23-2300-23-6 |
2.3 |
2300 |
23G |
6 |
≥2.8 |
For other needle model, please consult us for more information.
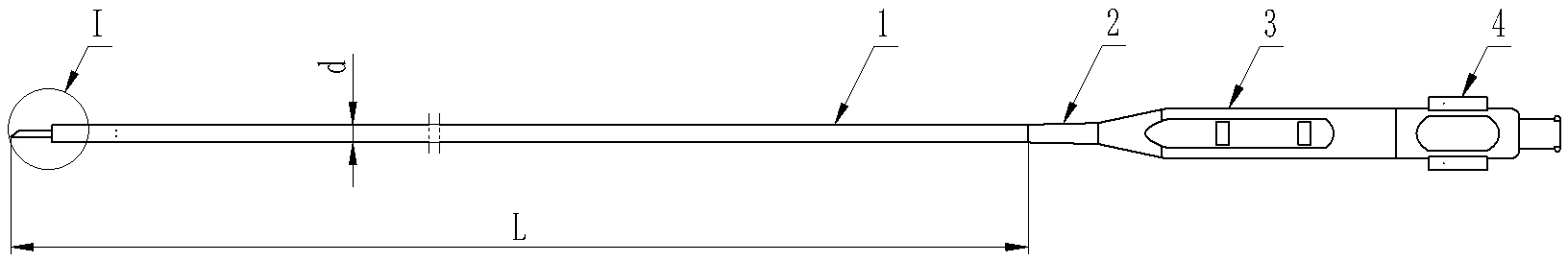
I Enlarged sectional view
I Enlarged sectional view
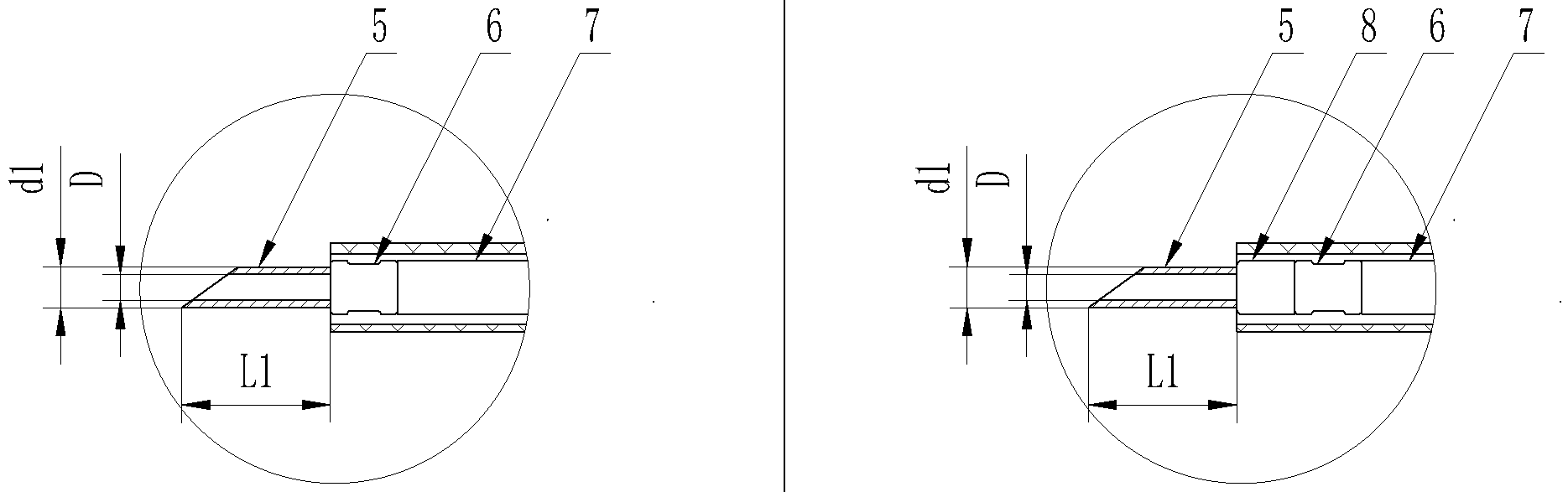
Type A
Type B
1.Outer tube 2.Protective tube 3.Front handle 4.Back handle
5.The needle 6.The needle casing 7.Inner tube 8.The metal cap
Structure diagram of disposable endoscopic injection needles.
Instructions
1. Aseptic packaging inspection: Check aseptic packaging for breakage, loose seal or water immersion, etc.
2. Appearance inspection: Take the product out of the package, check whether the outer pipe and inner pipe are bent, kinked, damaged, etc., check whether the parts of the product fall off, loose, damaged, etc.
3. Operation check: Expand and straighten the outer tube, imitate the operation, first push the back handle into the front handle, until the click sound is heard, confirm that the needle tube extends from the front end of the outer tube. Then pull the back handle back to the end until you hear the click and ensure the needle turns back into the outer tube.
4. Injection check: Push the back handle to the end to extend the needle out from the front end of the outer tube. Firstly, connect the syringe that has been filled with liquid with the conical joint of the rear handle, and then push the syringe piston to drain the air in the inner tube until the liquid flows out at the point of the needle, and make sure that there is no liquid leakage beyond the point.
5. Pull back the handle to the end to draw the needle tube back into the outer tube. Insert the outer tube into the forceps hole of the endoscope and slowly advance the outer tube until the end of the outer tube head enters the endoscopic field of view.
6. Select the appropriate injection site through endoscope and keep the lens position. Push the rear handle into the front handle assembly until the ‘click’ sound. Confirm that the needle tube is extended from the front end of the guide head. Then insert the needle into the target tissue and push the syringe piston for injection operation.
7. After the operation, pull back the handle and the end of the handle until the click sound.
Then, pull the needle back into the guide and remove the product from the endoscope.
8. Postoperative treatment: Dispose of this product according to the biological hazard medical waste material management standard of the institution.
High Quality Guaranteed
Assembly Process:
2000+Sq. 100,000 Grade Cleaning Workshop.
Inspection Process:
Physical and chemical laboratories with advanced instruments, ensuring the ultra quality of each product.

Certificate
Product detail pictures:



Related Product Guide:
Pioglitazone Hcl Market by 2019-2024: Suppliers, Challenges, CAGR, Trends, Size & Shares, Countries, Revenue | Endoscope Biopsy Forceps
Cytology Brush Market Growing Popularity and Emerging Trends | Boston Scientific, Olympus America, Cook Medicals, EndoChoice, Medi-Globe GmbH | Cylindrical Anoscope
Our progress depends around the innovative machines, great talents and consistently strengthened technology forces for OEM manufacturer Disposable For Biopsy Forceps - Disposable cytology brush – Chenmao, The product will supply to all over the world, such as: Guinea , Nigeria , Canberra , Due to the stability of our items, timely supply and our sincere service, we are able to sell our merchandise not only over the domestic market, but also exported to countries and regions, including the Middle East, Asia, Europe and other countries and regions. At the same time, we also undertake OEM and ODM orders. We will do our best to serve your company, and establish a successful and friendly cooperation with you.
Adhering to the business principle of mutual benefits, we have a happy and successful transaction, we think we will be the best business partner.






